
Development Services and CMO
Eckert & Ziegler Medical is your reliable partner for research, development and routine production of radiopharmaceuticals and medical devices.
Combining the know-how from several successful projects for start-ups and enterprises in the pharmaceutical industry with decades of experience with medical radioisotopes, equipment for their handling and quality control, we offer the full range of services from early development, including process development and scale-up, CMC manufacturing, product release, stability programs and packaging, to the manufacturing of products for the clinical phase and commercial stage.
Our Products and Services
With more than 30 years in the radiation therapy business, Eckert & Ziegler Medical also focuses on the provision and handling of radiopharmaceuticals.
Apart from support for and actual development of radiopharmaceuticals, kits and more in our GMP-laboratories, we offer design, automation and quality control for processes with our state-of-the-art equipment. For independent projects we provide you with customized hot cell and processing solutions. Alternatively, our GMP-suites can be temporarily rented to reach dedicated project milestones. Alternatively, the complete manufacturing process, including regulatory support, will be handled by our team as part of contract manufacturing. Our experts for logistical challenges and packaging will guarantee a safe transfer to your customers.
Whether you are looking for a European, a North American, an Asian or even an global solution, with locations in Germany (Berlin and Brunswick), the USA (Wilmington, MA) and the planned new site in China (Jintan), we are your ideal production and development partner.
We are looking forward to discussing your project!
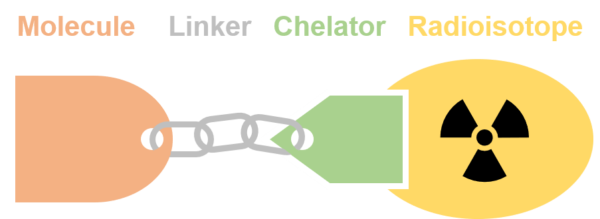
Radiochemistry Development and CMC
Projects in early development and preclincal stages often require radiochemistry development support as well as Chemistry, Manufacturing and Controls (CMC) expertise.
With a customer-specified biomolecule and radioisotope as the basis, Eckert & Ziegler is able to develop a robust radioconjugate (radiolabeled molecule) by selecting a suitable chelator and its position in the ligand, defining a linker and testing the compound for retention of its characteristics. Testing includes various aspects such as different temperatures, activity levels, time frames and others.
To guarantee consistency between batches, the radioconjugate will be optimized and methods for manufacturing and testing of the potential radiopharmaceutical will be defined. All results are properly recorded in a comprehensive report, which can then be used as a basis for following development and clinical stages including the registration of a radiopharmaceutical.
Process Automation and Quality Control
Whether the knowledge and equipment for the handling of radiopharmaceutical products is completely missing or a manual process has to be transferred to an automated solution e.g. for upscaling, Eckert & Ziegler provides the required services. With our production systems based on the Modular-Lab technology we customize synthesis equipment, disposable cassettes and software according to your needs to develop the ideal automated process. In addition, the wide range of radiochromatography systems allows the development of quality control standards specifically for your application.
We also offer product release, stability programs and packaging to guarantee a smooth transfer to clinical and commercial production.
Contract Manufacturing (CMO)
Eckert & Ziegler serves as a Contract Manufacturing Organization (CMO) for clinical as well as commercial supply for projects using alpha, beta or gamma radiation. Whether you require dedicated radionuclides in highest quality, radiolabeled pharmaceuticals or the manufacturing of medical devices, our team provides the ideal solutions customized to your pre-defined specifications. Within the range of services, we also offer regulatory support, e.g. the application of manufacturing authorizations and share our experience in registration processes.
CMO for Clinical Phases I/II and III
A large number of radiopharmaceutical substances from international pharmaceutical companies are in advanced clinical trials, some of them for broad indications such as prostate cancer. With our experience and capabilities in clinical scale manufacturing for phases I, II and III, the existing production facilities as well as our comprehensive know-how in radiopharmaceuticals, we are your preferred contract manufacturing partner for clinical phases. All our facilities are equipped for GLP and cGMP compliant production.
CMO for Commercial Supply
Eckert & Ziegler also serves as a CMO for commercially oriented customers with radiopharmaceuticals and medical devices beyond clinical phases. In addition to already existing infrastructure, our portfolio of spaces and buildings as well as possibilities for extension, enables us to establish fully customized GLP and cGMP-compliant production facilities within a short time frame. Supported by our internal expertise and capabilities in process development, manufacturing, regulatory aspects and the installation of manufacturing environments such as hot cells, the full spectrum of necessary services for the production of your product will be covered.
GMP Suites
With the advancing number of cutting-edge start-up companies as well as the introduction of pharmaceutical enterprises with non-radiation focus to the nuclear medicine and radiopharmaceutical market the demand for services for both, imaging and therapy products significantly increases.
Our GMP suite concept offers companies without the necessary infrastructure the possibility to rent equipped space and experienced personnel for the completion of testing and project milestones in all development and clinical phases. Our clean rooms will be equipped according to the customer requirements with hot cells, radiosynthesis, quality control and further equipment. All laboratory spaces for radiopharmaceutical services are operated under GLP and cGMP conditions. Our experts will be consulted to guarantee the optimal environment for your needs.

Customized Hot Cell Solutions
In the work with radionuclides, radiation shielding and continuous optimization of processes play a crucial role. For manufacturers of radiopharmaceuticals and suppliers of alpha-, beta- and gamma-emitting radioisotopes with own facilities Eckert & Ziegler Medical offers customized hot cell solutions, in-cell equipment and process development to facilitate daily business.
With the experience from three decades and several sophisticated projects we offer the following solutions tailored to your needs:
- Medical Applications
- Radiopharmaceutical Production, Labeling and Dispensing
- Research and Development
- Radionuclide Production
- Non-Medical Applications
- Material Testing
- Research and Development
- Radionuclide Production e.g. for industrial sources
- Reprocessing and Disposal

References
The following projects have been realized apart from several undisclosed development and manufacturing agreements and internal projects:
- Supply of Y-90 to Sirtex Medical for use in their Sirtex SIR-Spheres(R) Y-90 resin microspheres
- Supply of Th-228 to Alpha Tau Medical for the production of their innovative therapy Alpha DaRT
- Joint-Venture with Chengdu New Radiomedicine Technology Co. for the production of Y-90 based products
- Manufacturing for clinical supply of Bayer‘s innovative Targeted Thorium Conjugates (TTCs) in Europe
- Development of an automated process for the routine production of Curium‘s Ioflupane (123I) tracer STRIASCAN™
- Development and validation of the pharmaceutical production process for Curasight
- Support of Octreopharm Sciences Ipsen with laboratory space and equipment
Further projects include kit development, CMC studies, Contract Manufacturing and others.
Please contact us to find out more and discuss your projects!
Contact
Medical Segment
Robert-Rössle-Str. 10
13125 Berlin
Germany